Formulation and Optimisation of Mucoadhesive Buccal Films: A Pharmacokinetic Study in Rabbit Models
Keywords:
- Mucoadhesive buccal film, 3² factorial design, pharmacokinetics, bioavailability, systemic drug delivery, Metoprolol tartrate, rabbit model
Abstract
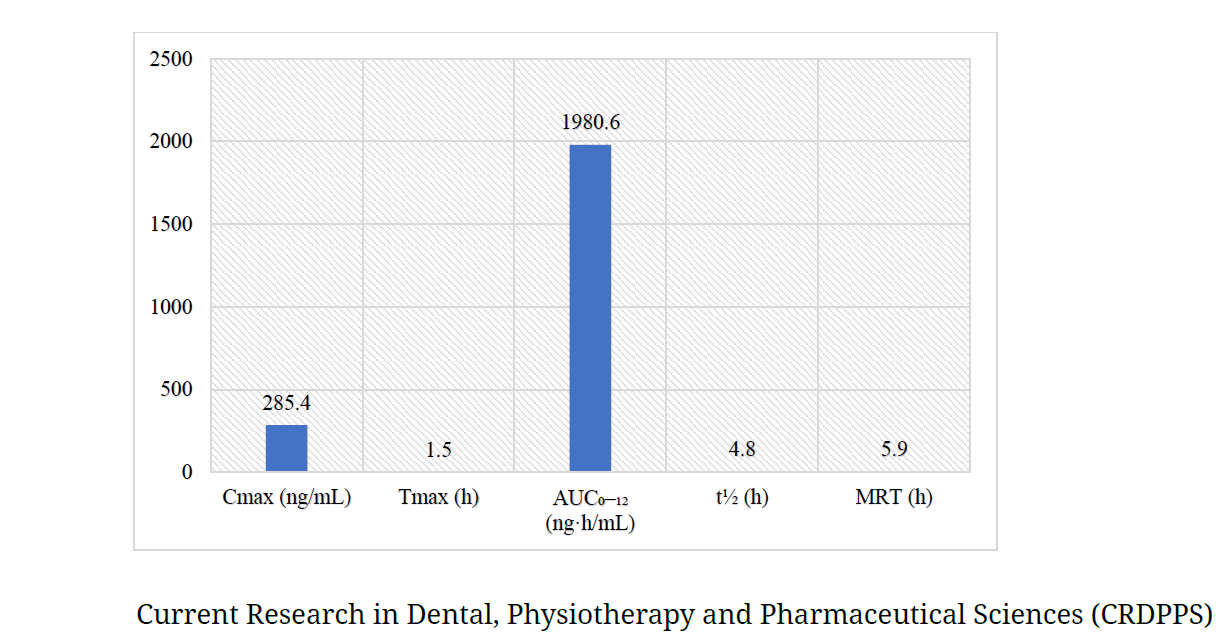
Mucoadhesive buccal films have now become a favorable substitute for conventional oral drug administration by facilitating direct systemic absorption, avoiding hepatic first-pass effect, and maximizing bioavailability. The present research was carried out to develop, optimize, and characterize mucoadhesive buccal films through a 3² factorial design by employing different concentrations of hydroxypropyl methylcellulose (HPMC) and PEG-400. The films were developed by using the solvent casting technique and tested for major physicochemical characteristics such as thickness, surface pH, drug content, and in vitro drug release. The optimized formula (F5) exhibited outstanding physicochemical properties and controlled drug release (94.6% at 8 hours). Pharmacokinetic assessment was carried out in six healthy New Zealand white rabbits with comparison between buccal film and conventional oral tablet. The buccal film showed better pharmacokinetic behavior with much higher Cmax (285.4 ng/mL), lower Tmax (1.5 h), larger AUC₀–₁₂ (1980.6 ng·h/mL), and longer half-life (4.8 h) than the oral tablet. Statistical evaluation by independent t-tests also revealed significant enhancements in systemic bioavailability and onset of action. These results indicate that the optimized mucoadhesive buccal film is a good drug delivery system for therapeutic improvement.